About Janusmed Sex and Gender
Introduction
Janusmed Sex and Gender is a knowledge database providing structured evidence-based information on sex and gender aspects of drug treatment. The purpose is to provide support for improved drug therapy by facilitating the right choice of drug and the right dosage, related to the patient’s sex. The text documents are based on substance names (generic names).
The knowledge database is primarily intended to be used by physicians and healthcare professionals. Please note that the information in the knowledge database is general and does not replace medical, clinical, or pharmaceutical assessment. The information should not be considered as treatment guidelines. The individual patient’s physician is responsible for the patient’s drug treatment. Therefore, patients who have questions are referred to their responsible physician.
About the content
Optimization of drug prescription and drug treatment in men and women is dependent on analyses of diseases and health with a sex and gender perspective. More evidence of great importance on differences between men and women in drug treatment, both from a biological and a sociological perspective, is continuously found.
In this context, the term ‘sex differences’ refers to biological differences, and the term ‘gender differences’ refers to differences related to attitudes in society as well as economic, ethnic, and socio-psychological differences.
Read more: Overview of sex and gender differences in drug treatment
The text documents in Janusmed Sex and Gender primarily contain information on biological sex aspects regarding pharmacokinetics, effects, and adverse effects. In addition, to some extent information on gender aspects e.g., disease prevalence, drug use, and adverse event reporting. The sex and gender differences in the text documents are statistically significant unless otherwise stated.
Please note that Janusmed Sex and Gender does not include information on possible drug effects on the fetus or if drug treatment is compatible with breastfeeding. For related information, please consult the knowledge databases Janusmed Drugs and Birth Defects (in Swedish, Janusmed fosterpåverkan) and Janusmed Breastfeeding (in Swedish, Janusmed amning).
The information in the knowledge database is based on systematic searches in scientific literature, other databases, and handbooks. The literature search follows a template (SOP) and a checklist to ensure that the authors follow the same systematic process and have enough information to write about substances that not yet have been included in the knowledge database, and to update existing text documents. The text documents are written by pharmacists or physicians. Before publication, all texts are reviewed by specialists in clinical pharmacology and approved by a senior professor. Updates of the content are done continuously. If necessary, the medical content is reviewed by clinical experts from Region Stockholm’s Drug and Therapeutic Committee’s expert groups within relevant therapy area. In addition, the Swedish Medical Products Agency or its American counterpart, the Food and Drug Administration, are consulted if necessary.
Most drugs in the database are also recommended in The Wise List of Region Stockholm. In addition, also other commonly used drugs are included.
In case you can’t find the drug substance you’re looking for, contact us at e-tjanster.hsf@regionstockholm.se.
Regarding the words ‘woman’ and ‘man’ in our texts
We write women/girls and men/boys in general in our texts. This is because the knowledge base in the database is based on studies where the population has been divided according to these categories. The knowledge database only covers studies on humans and studies that included individuals with the biological sexes woman and man (or girl and boy) because there is a lack of sufficient data to analyze non-binary gender identities.
Classification
All drug substances in Janusmed Sex and Gender are assigned to one of four classification categories. The classification is based on the amount of evidence for sex differences for the substance found in the literature searches.
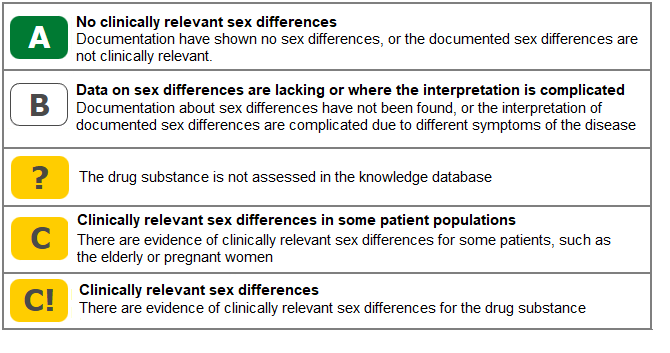
Validation of scientific evidence regarding drugs and sex/gender
The information about drugs and sex/gender is based on structured literature searches in PubMed. The selection of articles for extensive review has been made according to the principles of evidence-based medicine. Thus, the knowledge compilation is based primarily on meta-analysis or well-done randomized clinical trials. When these are lacking, observational studies have been used.
Few studies are designed in order to directly study sex and gender differences in drug utilization. The information has therefore been collected from many different sources. Thus, a fair grading of evidence becomes difficult to implement, so we opted not to enter evidence level of this knowledge database Janusmed Sex and Gender. General levels of evidence grading applied by the Swedish Medical Products Agency (MPA), Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU), and The National Board of Health and Welfare are available on the agencies’ websites.
It is important to emphasize that the evidence grade not only depend on the study type, but also how well-done the study is. A well-designed observational study can provide a higher evidence grade that a bad (or too small) clinical trial. Generalizability to actual medical care is also higher in observational studies because patients participating in clinical trials often differ from those receiving the drug when it’s available on the market, due to clinical trials’ narrow inclusion and exclusion criteria.
Sender and responsibilities
The information on this website is produced in collaboration between the Health and Medical Care Administration in Region Stockholm, Clinical Pharmacology at Karolinska University Hospital, and the Centre for Gender Medicine at Karolinska Institutet.
Responsible for the medical content are Clinical Pharmacology at Karolinska University Hospital and senior professor Karin Schenck-Gustafsson at Centre for Gender Medicine, Department of Medicine, Karolinska Institutet.
The Janusmed website is owned and operated by the Health and Medical Care Administration in Region Stockholm, Sweden.
Articles about the knowledge database
Sex and gender differences in drug treatment: experiences from the knowledge database Janusmed Sex and Gender.
Karlsson Lind L et al. Biol Sex Differ. 2023 May 12;14(1):28.
Sex differences in drugs: the development of a comprehensive knowledge base to improve gender awareness prescribing.
Karlsson Lind L et al. Biol Sex Differ. 2017 Oct 24;8(1):32.
Publications and dissertations within the topic
Sex and gender aspects in drug utilization.
Desirée Loikas. Karolinska Institutet. 2021.
Sex differences in cancer risk and survival.
Cecilia Radkiewicz. Karolinska Institutet. 2019.
Sex differences in adverse drug events from cardiovascular medicines in routine care.
Diana Rydberg. Karolinska Institutet. 2019.
Depression among adolescents and young adults: social and gender differences.
Therese Wirback. Karolinska Institutet. 2018.
Antiepileptic drug utilization: need of sex-specific information and decision support.
Linnéa Karlsson Lind. Karolinska Institutet. 2018.
Sex and gender differences in patients undergoing ablation of atrial arrhythmias.
Carina Carnlöf. Karolinska Institutet. 2018.
Diagnostic precision and sex differences in quantitative cardiovascular magnetic resonance.
Jannike Nickander. Karolinska Institutet. 2018.
Does patient’s sex influence treatment in primary care? Experiences and expressed knowledge among physicians – a qualitative study. Loikas D et al. BMC Fam Pract. 2015;16(1):137.
Severe sepsis epidemiology and sex-related differences in inflammatory markers.
Sofie Jacobson. Umeå University 2014.
Treatment of hypertension in men and women.
Charlotta Ljungman. Göteborg University. 2014.
Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. Loikas D et al. BMJ Open. 2013;3(5).
Stora könsskillnader i användningen av läkemedel (in Swedish)
Loikas D et al. Läkartidningen. 2011;108:40
Links
E-learning
Free online course about sex- and gender-related differences in health and disease in key disciplines, such as immunology, cardiovascular disease, and mental health. Developed by FDA and NIH.
CAS program in Sex- and Gender-Specific Medicine
Part-time course for physicians and medical researchers, University of Bern and Zürich University.
Gendered Innovations
Knowledge and research center, Stanford University
eGender Medicine
Web course in gender medicine, Institute of Gender in Medicine (GIM), Charité, Universitätsmedizin Berlin.
Drug Trials Snapshots
Demographic data from clinical trials of newly approved drugs by Food and Drug Administration (FDA).
Handbook of Clinical Gender Medicine
Handbook in gender medicine. Schenck-Gustafsson et al (eds). Karger; 2012.
Contact us
If you have questions or comments, you can contact us at e-tjanster.hsf@regionstockholm.se. We will respond to your query as soon as possible.
Healthcare professionals in Region Stockholm with individual questions concerning a patient and ambiguity, or lack of information in the knowledge database, can contact the medicine information center Karolic, Karolinska University Hospital, Stockholm: karolic.karolinska@regionstockholm.se.
Healthcare professionals in other parts of Sweden are referred to their regional medicine information center; svelic.nu.
The information in this knowledge database is aimed only for healthcare professionals. Patients who have questions are referred to their physician.
Last updated October 23, 2024